Abstract
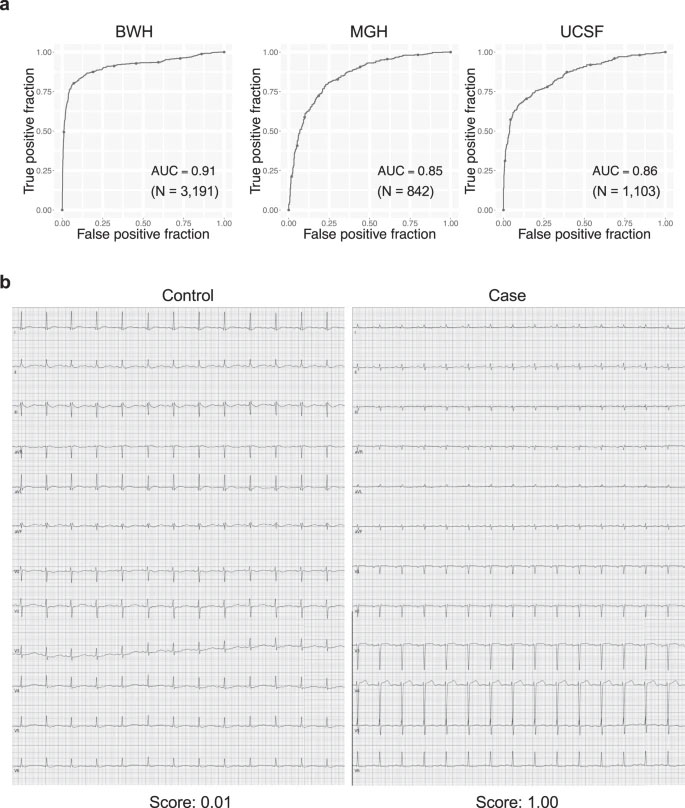
Patients with rare conditions such as cardiac amyloidosis (CA) are difficult to identify, given the similarity of disease manifestations to more prevalent disorders. The deployment of approved therapies for CA has been limited by delayed diagnosis of this disease. Artificial intelligence (AI) could enable detection of rare diseases. Here we present a pipeline for CA detection using AI models with electrocardiograms (ECG) or echocardiograms as inputs. These models, trained and validated on 3 and 5 academic medical centers (AMC) respectively, detect CA with C-statistics of 0.85–0.91 for ECG and 0.89–1.00 for echocardiography. Simulating deployment on 2 AMCs indicated a positive predictive value (PPV) for the ECG model of 3–4% at 52–71% recall. Pre-screening with ECG enhance the echocardiography model performance at 67% recall from PPV of 33% to PPV of 74–77%. In conclusion, we developed an automated strategy to augment CA detection, which should be generalizable to other rare cardiac diseases.
Introduction
Cardiac amyloidosis arises from deposition of misfolded proteins in the heart muscle, which results in a restrictive-type cardiomyopathy, and commonly progresses to heart failure, conduction system disease, and cardiac death. Cardiac amyloidosis is subclassified based on the specific protein involved, with the major subtypes being transthyretin amyloidosis (ATTR cardiac amyloidosis), caused by misfolding of the transthyretin protein, and light chain amyloidosis (AL cardiac amyloidosis), caused by accumulation of immunoglobulin light chains1. Cardiac amyloidosis was previously believed to be rare, but recent reports have suggested that it is largely underdiagnosed2,3,4,5,6. The imperative of identifying patients has dramatically increased with the advent of therapies for specific forms of cardiac amyloidosis7,8,9,10,11.
The clinical manifestations of cardiac amyloidosis—including conduction system disease, vitreous opacity, carpal tunnel syndrome, orthostatic hypotension, polyneuropathy, spinal stenosis, kidney dysfunction, atrial fibrillation, heart failure—are also commonplace in aging, thus making detection challenging. These signs and symptoms are distributed across multiple organs and tissues (and therefore medical disciplines), and the probabilistic weighting of so many different features is forbidding, even in the unlikely event that all of the relevant exam findings, medical history details and diagnostic test results were available to a given practitioner. Furthermore, definitive diagnostic tests for cardiac amyloidosis—which include tissue biopsy and some forms of radionuclide scintigraphy—are costly and have associated risk, and thus are not plausible as screening approaches12.
Cardiac amyloidosis nonetheless has predictive features captured by less expensive and more widely available diagnostic modalities such as electrocardiography13,14,15,16 (ECG) and echocardiography17,18, but the features themselves are not highly specific and thus often missed. Also, some of the recently highlighted echocardiographic features require providers to master specialized software packages19, which are time-consuming to use and therefore tend to be employed in practice only after the disease is suspected. A truly generalizable detection strategy should require no specialized acquisition or processing and should rely on only widely available input data. However, the low existing prevalence of the disease places high demands on model performance to reduce the rate of costly false positives, something that has not been achieved to date.
Here, we show a human-interpretation-free machine learning pipeline that accurately detects cardiac amyloidosis using a combination of ECG and echocardiography across multiple institutions.
Read the whole article at www.nature.com